Under U.S. law, whether a product is a cosmetic or a drug depends on the intended use of the product. Different laws and regulations apply to cosmetics and drugs.
Intended Use
In the U.S., the FDA regulates toothpaste as drugs or cosmetics, depending on their ingredients and purpose. Many other countries also classify toothpaste in the same way as the U.S.
- Toothpaste are drugs if they contain fluoride, are intended to prevent or lessen diseases like tooth decay or affect the structure of the body or how it functions.
- Toothpaste that does not contain fluoride and that claims only to cleanse teeth are considered cosmetics.
- When a toothpaste meets the definition of both a cosmetic and a drug or when a toothpaste has two intended uses, the toothpaste is both a cosmetic and a drug, for example, fluoride toothpaste.
Laws and Regulations Different for Cosmetics and Drugs
The laws and regulations for cosmetics and drugs differ in the areas of approval, good manufacturing practice (GMP), registration, and labeling.
Approval Requirements
Under the FD&C Act, cosmetic toothpastes and their ingredients, with the exception of color additives, do not require FDA approval prior to marketing.
However, drug toothpastes must comply with the appropriate monograph for an OTC drug, these “monographs” specify conditions under which OTC drug ingredients are generally recognized as safe and effective and are not mislabeled.
GMP Requirements
Good Manufacturing Practice (GMP) is an important factor in ensuring that cosmetics are not adulterated or mislabeled.
The FDA provides guidelines for cosmetic GMP (see “Good Manufacturing Practice (GMP) Guidelines/Inspection Checklist“), but there are no regulations that specify specific GMP requirements for cosmetics. The guidelines refer to information on building and facilities, equipment, personnel, raw materials, production, laboratory controls, records, labeling, complaints, and others.
In contrast, the law requires strict adherence to GMP requirements for drugs, and there are regulations that specify the minimum requirements for current drug GMP [Title 21 of the Code of Federal Regulations (CFR), parts 210 and 211], the non-compliance of which can lead to adulteration of drugs [FD&C Act, sec. 501(a)(2)(B)].
Registration Requirements
In the past, the FD&C Act did not require cosmetic companies to register their establishments or list their product formulations with the FDA.
FDA maintains the Voluntary Cosmetic Registration Program, or VCRP, for cosmetic establishments and formulations, however, FDA stopped accepting and processing both electronic and paper submissions of cosmetics establishments and products to the VCRP on March 27, 2023.
Now, Modernization of Cosmetics Regulation Act of 2022, or MoCRA provides the FDA with new authorities, manufacturers, and processors must register their facilities with the FDA and renew their registration every two years. Besides, a responsible person must list each marketed cosmetic product with the FDA, including product ingredients, and provide annual updates.
The FDA initially required that stakeholders should plan to register and list by the statutory deadline of Dec. 29, 2023; however, the FDA said in November that it could delay this by six months, until the end of June 2024.
The good news is that MoCRA exempts certain small businesses from GMP, registration, and product listing requirements.
In contrast, drug companies must register their facilities with the FDA and list their drugs [FD&C Act, sec. 510; 21 CFR 207]. See Drug Registration and Listing System (DRLS and eDRLS).
Labeling Requirements
Cosmetic toothpaste and drug toothpaste have different labeling requirements, which are listed separately below.
Cosmetics Labeling Guide
On the principal display panel:
- the name of the product,
- the statement of identity,
- net contents,
- there may be statements such as, “Warning – The safety of this product has not been determined”,
- and others.
On the information panel:
- the name and address of the manufacturer or distributor,
- the list of the ingredients (only on the outer container),
- the warning statement(s),
- any direction,
- and others.
| Front Panel | Information Panels |
|---|---|
| Directions for safe use | |
| Warnings | |
| Name of Product | Name and Place of Business |
| Net Quantity of Contents | |
| Any Other Required Information |
| Principal Display Panel: Name of product Identity § 740.10 warning Net quantity of contents | Information Panels: Directions for safe use Warnings Name and place of business Ingredient declaration Any other required information |
|---|
Drugs Labeling Guide
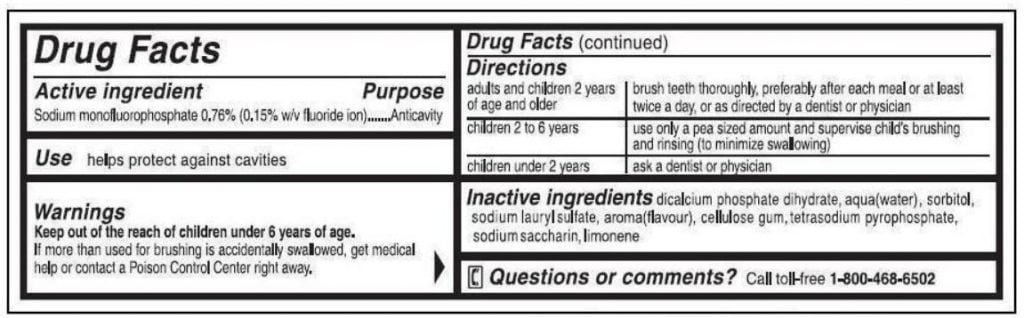
Over-the-counter toothpaste that is not sold as a cosmetic must be labeled in the “Drug Facts” format, which means a standard “box” format on the side or back panels of the outer carton or on the tube of toothpaste if there is no outer carton. This format is easy to recognize:
– The panels are labeled with the heading “Drug Facts.”
– Information in the panel is printed in a single color against a white or contrasting background.
– Information in the box is separated by horizontal lines.
– Information is listed under the headings, and in this order:
1 Active ingredient(s)
2 Use
3 Warnings
4 Directions
5 Other information
6 Inactive ingredients
7 Questions